Source – Arsenic (As) is not easily dissolved in water, therefore, if it is found in a water supply, it usually comes from mining or metallurgical operations or from runoff from agricultural areas where materials containing arsenic were used as industrial poisons. Arsenic and phosphate easily substitute for one another chemically, therefore commercial grade phosphate can have some arsenic in it. Arsenic is highly toxic and has been classified by the US EPA as a carcinogen. The current MCL for arsenic is 0.05 mg/l which was derived from toxicity considerations rather than carcinogenicity.
Treatment – If in an inorganic form, arsenic can be removed or reduced by conventional water treatment processes. There are five ways to remove inorganic contaminants; reverse osmosis, activated alumina, ion exchange, activated carbon, and distillation. Filtration through activated carbon will reduce the amount of arsenic in drinking water from 40 – 70%. Anion exchange can reduce it by 90 – 100%. Reverse Osmosis has a 90% removal rate, and Distillation will remove 98%. If the arsenic is present in organic form, it can be removed by oxidation of the organic material and subsequent coagulation.
Arsenic Chemistry
ArsenicTreatmentMTACFinalReport.pdf
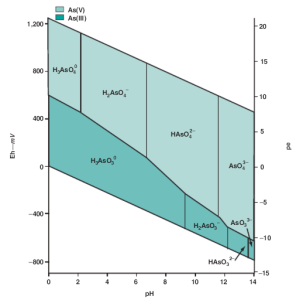
Arsenic in groundwater occurs in two oxidation states, As(III) (arsenite) and As(V) (arsenate).
Arsenious acid (H3AsO3) has a pKa value of 9.2, so in the pH range of most groundwaters there is
relatively little of its conjugate base H2AsO3
– (Figure 4). The sum of concentrations of H3AsO3
and H2AsO3
– is denoted As(III). Arsenic acid (H3AsO4) has pKa values of 2.7, 6.8, and 11.5,
which are similar to those of phosphoric acid. In the pH range of groundwater the concentrations
of H2AsO4
– and HAsO4
2- are much greater than those of H3AsO4 and AsO4
3- (Figure 5). The sum
of concentrations of H3AsO4, H2AsO4
-, HAsO4
2-, and AsO4
3- is denoted As(V). Although
methylated forms of arsenic are sometimes found in surface waters, they have only rarely been
found in groundwater (Irgolic, 1982; Chatterjee et al., 1995), except in cases of gross
contamination by herbicides (Holm et al., 1979). Shraim et al. (2002) did find low concentrations
(< 2 mg/L) of methylated species in groundwater from West Bengal, although the inorganic
arsenic concentration in those samples was extremely high (> 300 mg/L).
In most published studies of arsenic speciation, both As(III) and As(V) were found and the less
abundant species was at least 2% of the total arsenic (Matisoff et al., 1982; Ficklin, 1983; Welch
et al., 1988; Chen et al., 1995; Smedley, 1996; Smedley et al., 1996; Boyle et al., 1998; Yan et
al., 2000). In these studies, As(III) was generally predominant under reducing conditions while
As(V) was predominant under oxidizing conditions. Korte and Fernando (1991) found only
As(III) in shallow wells in an alluvial aquifer in Missouri.
Potenteil pH arsenic

Potenteil pH arsenic

Potenteil pH arsenic
 Mon Agora – Blog La parole est à moi
Mon Agora – Blog La parole est à moi
